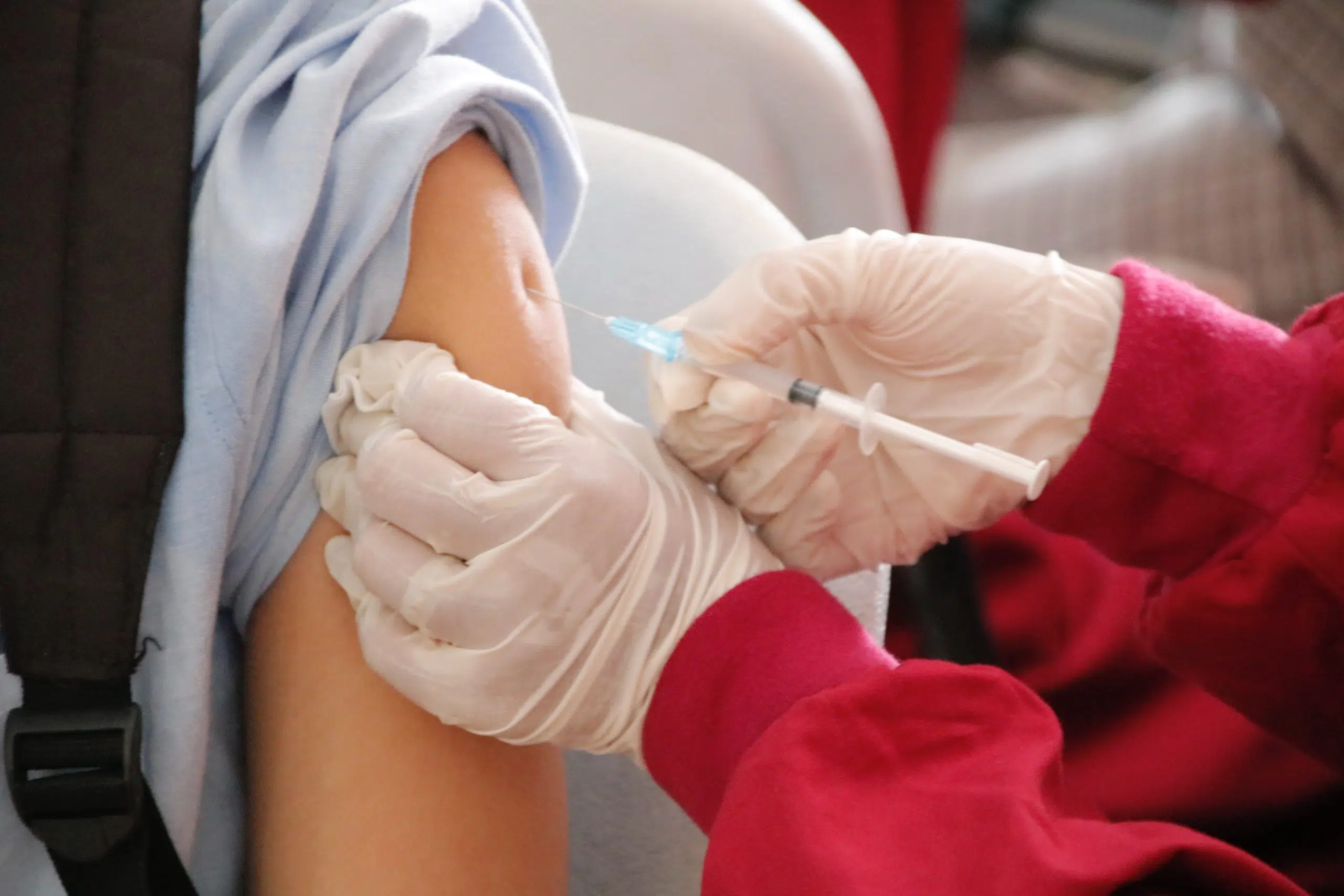
The FDA has approved updated COVID-19 booster shots to target the XBB 1.5 variant ahead of a potential winter uptick in infections. LSU Health New Orleans Infectious Disease specialist Dr. Fred Lopez says the vaccine is designed to offer continued protection against serious consequences from COVID-19.
“They’re major value really is in preventing complications including hospitalizations and death and that’s really why they’re really valuable parts of our armamentarium against COVID-19.”
The shots made by Moderna and Pfizer are approved for people 12 and older and under emergency use for children 6 months through 11 years old. Lopez says it’s still unclear which populations health officials will consider for the shots.
“A panel of advisors for the CDC will meet and make recommendations specifically for who should receive the vaccine.”
Over 95 percent of the U.S. population already has some level of COVID immunity according to the CDC. The Lopez says cases have increased in recent weeks and urges the public to consider the vaccination.
“To decrease the circulation of illness not just to the individual but its a public health issue. We do know some people are more vulnerable to complications and we want to prevent the transmission of these infections to those individuals in particular.”
Pfizer and Moderna will charge up to $130 dollars a shot, compared to $30 last year for the booster. The CDC advisory committee will meet today to determine how the vaccine should be used.





Comments